Cassava Sciences Announces Positive Top-Line Clinical Results in Phase 2 Study Evaluating Simufilam in Alzheimer’s Disease
January 24, 2023
- ADAS-Cog mean scores changed minimally over 1 year in patients with mild-to-moderate Alzheimer’s disease treated with open-label simufilam tablets.
- 47% of patients improved on ADAS-Cog over 1 year, and this group improved by 4.7 points. An additional 23% of patients declined less than 5 points on ADAS-Cog over 1 year, and this group declined by 2.5 points.
- Mild patients responded better than patients with moderate Alzheimer’s disease.
- Simufilam was safe, well tolerated.
AUSTIN, Texas, Jan. 24, 2023 (GLOBE NEWSWIRE) — Cassava Sciences, Inc. (Nasdaq: SAVA), a biotechnology company, today announced positive top-line Phase 2 results for simufilam, its oral drug candidate for Alzheimer’s disease dementia. This was an open-label safety study with exploratory efficacy endpoints. The study enrolled over 200 patients with mild-to-moderate Alzheimer’s disease (MMSE 16-26). Study participants were administered open-label simufilam tablets 100mg twice daily for 1 year or more. Endpoints were measured at baseline (study entry) and month 12.
Top-line Results – mean scores, baseline to month 12 (lower is better, except for MMSE):
- ADAS-Cog11 scores changed from 19.1 (±9.2) to 19.6 (±13.3)
- MMSE scores changed from 21.5 (±3.6) to 20.2 (±6.4)
- NPI10 scores changed from 3.2 (±4.6) to 2.9 (±4.6)
- GDS scores changed from 1.8 (±1.8) to 1.4 (±1.9)
Alzheimer’s is a degenerative disease of the brain. Over time, cognition progressively worsens in the mild-to-moderate stages of Alzheimer’s as the disease takes its toll. ADAS-Cog scores that change minimally (or improve) over 1 year is a highly desirable outcome in a clinical study of mild-to-moderate Alzheimer’s disease.
Figure 1 presents a model of historical declines on ADAS-Cog in patients with mild-to-moderate Alzheimer’s disease.
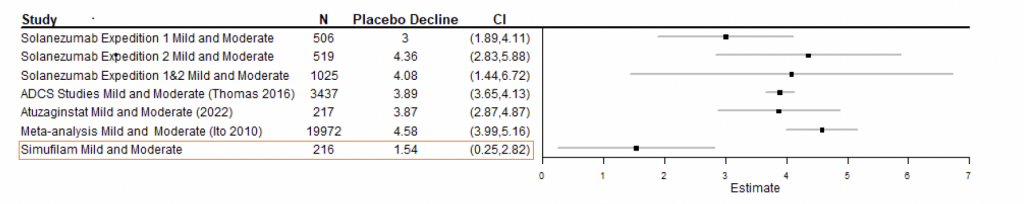
Figure 1: Statistical model of historical 1-year declines on ADAS-Cog, placebo vs simufilam treatment, mild-to-moderate disease.1
Response Analysis – baseline to month 12
- ADAS-Cog scores improved in 47% of patients; this group had a mean change of -4.7 (±3.8) points (lower is better).
- In an additional 23% of patients, ADAS-Cog declined less than 5 points; this group had a mean change of 2.5 (±1.4) points.
- Patients with an NPI10 score of zero increased from 42% to 54%, indicating reduced dementia-related neuropsychiatric symptoms.
“I’m very excited about these 1-year data,” said Remi Barbier, President & CEO. “They add strength and determination to our goal of helping people fight Alzheimer’s disease. Simufilam is an innovative drug candidate that we are developing methodically, one study at a time, and this open-label safety study served its purpose. Next up in 2023 are top-line clinical results of our Cognition Maintenance Study, which is a randomized, controlled trial.”
Analysis of Efficacy Endpoints
Efficacy outcomes were analyzed by an independent, outside biostatistical consulting firm led by Suzanne Hendrix, PhD. The pre-specified primary
efficacy endpoint was change in baseline on ADAS-Cog, a cognitive scale widely used in Alzheimer’s clinical research. Exploratory endpoints included the Mini-Mental State Examination (MMSE) to assess disease stage by cognitive impairment; the Neuropsychiatric Inventory (NPI10) to assess dementia related behavior; and the Geriatric Depression Scale (GDS). The Full Analysis Set (FAS) population (N=216) was used for the statistical analysis of efficacy endpoints.
Alzheimer’s is a progressive disease. Severity of disease is typically assessed by MMSE score. In this study, mild patients are MMSE 21-26; moderate patients are MMSE 16-20. Mild and moderate sub-groups showed notable differences on changes in ADAS-Cog mean scores, baseline to month 12 (lower is better):
- In the mild sub-group, ADAS-Cog scores improved, from 15.0 (±6.3) to 12.6 (±7.8)
- In the moderate sub-group, ADAS-Cog scores worsened, from 25.7 (±9.2) to 30.1 (±13.1)
“The data for simufilam are noteworthy,” said Suzanne Hendrix, PhD. “The improvement in ADAS-Cog over 1 year in mild patients taking simufilam is well outside the expected range of historic placebo decline rates from numerous other studies.”
Figure 2 presents a model of historical declines on ADAS-Cog in early disease and mild disease.
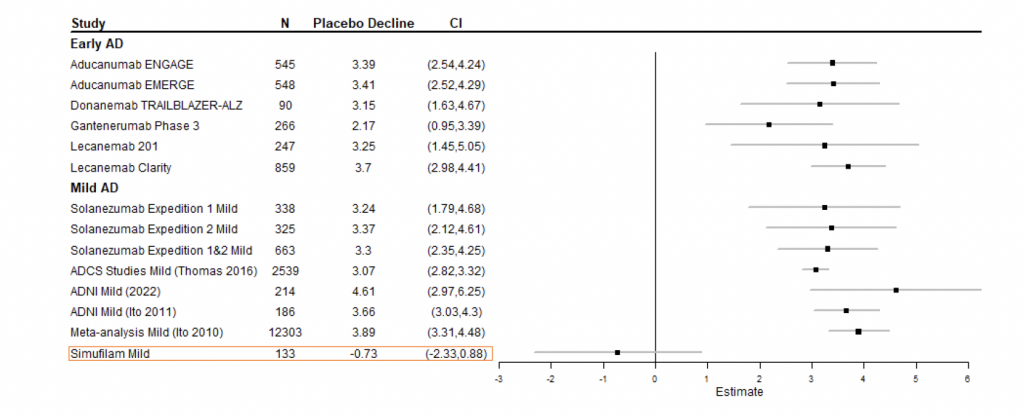
Figure 2: Statistical model of historical 1-year declines on ADAS-Cog, placebo vs simufilam treatment, in early disease and mild disease.2
Safety Data
Simufilam 100 mg twice daily was safe and well tolerated. There were no drug-related serious adverse events. Three treatment-emergent adverse events (TEAEs) occurred in 7% or more of study patients: COVID-19 (12%), urinary tract infection (10%) and headache (9%). Reported TEAEs are based on all study patients who received at least one dose of drug. The top three reasons for patient discontinuations were withdrawal of informed consent (N=14), adverse events (N=13) and patient non-compliance (N=7).
Biomarker Data
‘Research use only’, non-safety, exploratory biomarkers were analyzed from cerebrospinal fluid (CSF) collected from 25 patients in the open-label study who agreed to undergo a lumbar puncture at baseline and again after 6 months of treatment. CSF samples were analyzed blind by our academic collaborator at City University of New York (CUNY). We previously announced results of this bioanalysis in a press release dated July 29, 20213 (p-values shown below are baseline vs. 6-month levels by paired t-test):
- CSF biomarkers of disease pathology, t-tau and p-tau181, decreased 38% and 18%, respectively (both p<0.00001).
- CSF biomarkers of neurodegeneration, neurogranin and neurofilament light chain (NfL), decreased 72% and 55%, respectively (both p<0.00001).
- CSF biomarkers of neuroinflammation, sTREM2 and YKL-40, decreased 65% and 44% (both p<0.00001).
Of the 25 patients who provided 6-month CSF samples, 24 subsequently completed 1 year of treatment with open-label simufilam. This sub-set of patients improved -4.96 mean points on ADAS-Cog from baseline to month 12 (ad hoc analysis conducted internally; lower is better). We have not conducted further CSF sample analyses in the open-label study.
Chain of Custody for Clinical Data
Investigator sites collected clinical data from study patients. Sites entered their clinical data directly into an electronic data capture system managed by an independent, outside data management vendor. The data management vendor also maintains the clinical database. The data management vendor transmitted the clinical database directly to Pentara Corporation, an independent, outside consulting firm that specializes in complex statistical analysis of clinical trial results. Suzanne Hendrix, PhD, CEO of Pentara, has >150 peer-reviewed publications of clinical trial results and statistical approaches for clinical trials, many focusing on statistical methodology for Alzheimer’s disease.
Study Limitations
Data results from our open-label safety study do not constitute, and should not be interpreted as, regulatory evidence of safety or efficacy for simufilam in Alzheimer’s disease. Rigorous evidence for drug safety and efficacy is derived from one or more large, randomized, placebo-controlled studies. The open-label design and size of this study may introduce clinical or statistical bias or may generate results that may not fully distinguish between drug effects and random variation. Different methods of statistical analysis on clinical data from the same study may lead to objectively different numerical results. These and other statistical and clinical features of our open-label study add complexity or limitations to the scope of data interpretation.
‘Top-line data’ is a summary of the clinical data prior to the completion of a full and final audit or quality-control of the clinical database. We are communicating top-line data so that stakeholders may have timely access to a summary of the study’s findings prior to us receiving the final dataset. Final data may change from today’s top-line data. We expect to disclose the final dataset in a future medical conference or science publication.
On-going Phase 3 Studies with Simufilam
Cassava Sciences is currently evaluating simufilam tablets for Alzheimer’s disease dementia in two Phase 3 clinical studies. These are randomized, double-blind, placebo-controlled trials. The Phase 3 program is recruiting a total of approximately 1,750 patients with mild-to-moderate Alzheimer’s disease who also meet other study eligibility criteria. Both Phase 3 studies have received a Special Protocol Assessment (SPA) from the U.S. Food and Drug Administration. The Phase 3 studies are actively recruiting Alzheimer’s patients in over 100 clinical sites in the United States, Canada, Puerto Rico, South Korea and Australia.
About Simufilam
Simufilam is Cassava Sciences’ proprietary, small molecule (oral) drug candidate that restores the normal shape and function of altered filamin A (FLNA) protein in the brain. Cassava Sciences owns worldwide development and commercial rights to its research programs in Alzheimer’s disease, and related technologies, without royalty obligations to any third party.
About Cassava Sciences, Inc.
Cassava Sciences is a clinical-stage biotechnology company based in Austin, Texas. Our mission is to detect and treat neurodegenerative diseases, such as Alzheimer’s disease. Our novel science is based on stabilizing—but not removing—a critical protein in the brain. Our product candidates have not been approved by any regulatory authority, and their safety, efficacy or other desirable attributes have not been established.
For more information, please visit:
https://www.CassavaSciences.com
For More Information Contact:
Eric Schoen, Chief Financial Officer
(512) 501-2450, or eschoen@CassavaSciences.com
Cautionary Note Regarding Forward-Looking Statements:
This news release contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, relating to: our current expectations regarding the target patient enrollment numbers for our Phase 3 studies; our goals regarding timing for releasing top-line data for the Cognition Maintenance Study; comments made by our employees regarding simufilam and the treatment of Alzheimer’s disease; and potential benefits, if any, of our product candidates. These statements may be identified by words such as “may,” “anticipate,” “believe,” “could,” “expect,” “would”, “forecast,” “intend,” “plan,” “possible,” “potential,” and other words and terms of similar meaning.
Drug development involves a high degree of risk, and only a small number of research and development programs result in regulatory approval and commercialization of a product. Our interim data and analyses should not be relied upon as predictive of full study results for any of our studies. Our clinical results from earlier-stage clinical trials may not be indicative of full study results, or results from later-stage, or larger scale clinical trials, and do not ensure regulatory approval. You should not place undue reliance on these statements or any scientific data we present or publish.
Such statements are based largely on our current expectations and projections about future events. Such statements speak only as of the date of this news release and are subject to a number of risks, uncertainties and assumptions, including, but not limited to, those risks relating to the ability to conduct or complete clinical studies on expected timelines, to demonstrate the specificity, safety, efficacy or potential health benefits of our product candidates, the severity and duration of health care precautions given the COVID-19 pandemic, any unanticipated impacts of the pandemic on our business operations, and including those described in the section entitled “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2021, and future reports to be filed with the SEC. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from expectations in any forward-looking statement. In light of these risks, uncertainties and assumptions, the forward-looking statements and events discussed in this news release are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Except as required by law, we disclaim any intention or responsibility for updating or revising any forward-looking statements contained in this news release. For further information regarding these and other risks related to our business, investors should consult our filings with the SEC, which are available on the SEC’s website at www.sec.gov.
1 Figure 1: Forest plot meta-analysis model by Pentara Corporation. Data was sourced from randomized, controlled trials conducted by other sponsors in patients with mild-to-moderate Alzheimer’s disease.
2 Figure 2: Forest plot meta-analysis model by Pentara Corporation. Data was sourced from non-randomized studies (i.e., ADNI) and randomized, controlled trials conducted by other sponsors in patients with early (i.e., MCI + mild) and mild Alzheimer’s disease.
3 Statistical analysis on changes in CSF biomarkers was conducted internally; press release is available on-line: https://www.cassavasciences.com/node/15486/pdf.
Photos accompanying this announcement are available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/eff72f31-4f4c-4fae-94ca-50d517abcdb6
https://www.globenewswire.com/NewsRoom/AttachmentNg/595f0219-0f9e-43c6-b7a0-4b26dbcbd147



